#259 - Porphyrin nanocages, the Lotus Leaf, Ti4O7, Electrodialysis & more...
Water Water Everywhere...
Hey Readers,
This week we have some not-so-good news to get into, as well as a whole host of innovations. Let’s go!
News Roundup
The EWG did some research into cancer causing elements in water bodies and came to the realization that Manure contaminates water, which requires disinfection, and the process can trigger the formation of cancer-causing byproducts. To understand specifically how manure results in trihalomethanes ending up in water bodies, read more here.
Chinese water diplomacy is causing pain in India. Read more from the folks at Mongabay here. Or just watch their video here .
Innovations Roundup
Minimizing brine from desalination
Regular readers will know that discharge brine as a waste product is considered a big issue in desalination, not to mention a quantity of water and minerals that could have other uses. Enter a team from the University of Minnesota who have in their own words developed ‘technology could help desalination plants be more sustainable by reducing waste while using less energy.’ To eliminate brine waste, typically desalination plants concentrate brines by heating and evaporating the water, which is very energy intensive, or with reverse osmosis, which only works at relatively low salinity.
This research team went with electrodialysis. In this setup water flows into many channels separated by membranes, and each membrane has the opposite electrical charge of its neighbors. The entire stream is flanked by a pair of electrodes. The positive salt ions move toward the negatively charged electrode, and are stopped by a positively charged membrane. Negative ions move toward the positive electrode, stopped by a negative membrane. This creates two types of channels—one that both positive and negative ions leave and another that the ions enter, resulting in streams of purified water and concentrated brine. With their chemistry, the researchers can produce membranes that are ten times more conductive than relatively leak-proof membranes on the market today.
Highly efficient Ti4O7 films for water decontamination
A team at the Institut National de la Recherche Scientifique (INRS) in Quebec, Canada have developed a novel photothermal material that directly converts sunlight into heat with unprecedented efficiency. This is important because it allows for a range of possibilities, amongst which is manufacture of high-performance anodes for the decontamination of water containing persistent pollutants.
They did this by synthesizing a titanium oxide (Ti4O7) powder, that can then be pressed to form pellets, or electrodes of a few centimeters in size. They used a technique known as “magnetron sputtering” (or RF-magnetron plasma) to deposit thin coatings of this material on to a substrate. This thin film deposition technique is commonly used in the semiconductor industry, and in this application allowed for a Ti4O7 coating of a few hundred nanometers thickness.
Lotus Leaf inspired desalination
A team at the Indian Institute of Technology, Bombay, have developed a new material that can facilitate water desalination. They based this design on the lotus leaf that floats on water but also absorbs water as needed for the plant to grow. The device is called a Dual-Sided Superhydrophobic Laser-Induced Graphene (DSLIG) evaporator. The device has the potential for large-scale applications as it can be heated by both solar energy and by electricity if needed.
The researchers fabricated DSLIG by coating a layer of a polymer called polyvinylidene fluoride (PVDF) on one side of a thin layer of another polymer polyether sulfone or PES. Graphene was then engraved on the PVDF polymer side of the material using laser-based engraving technology. This laser-induced graphene (LIG) interfacial evaporators has two distinct sides formed by the two polymers. The PES doesn’t repel water, but it is essential to prevent the evaporator from breaking easily. So with the PES as a substrate ensured mechanical stability, while the PVDF layer contributed to the hydrophobic (water repelling) characteristics necessary for efficient evaporation processes.
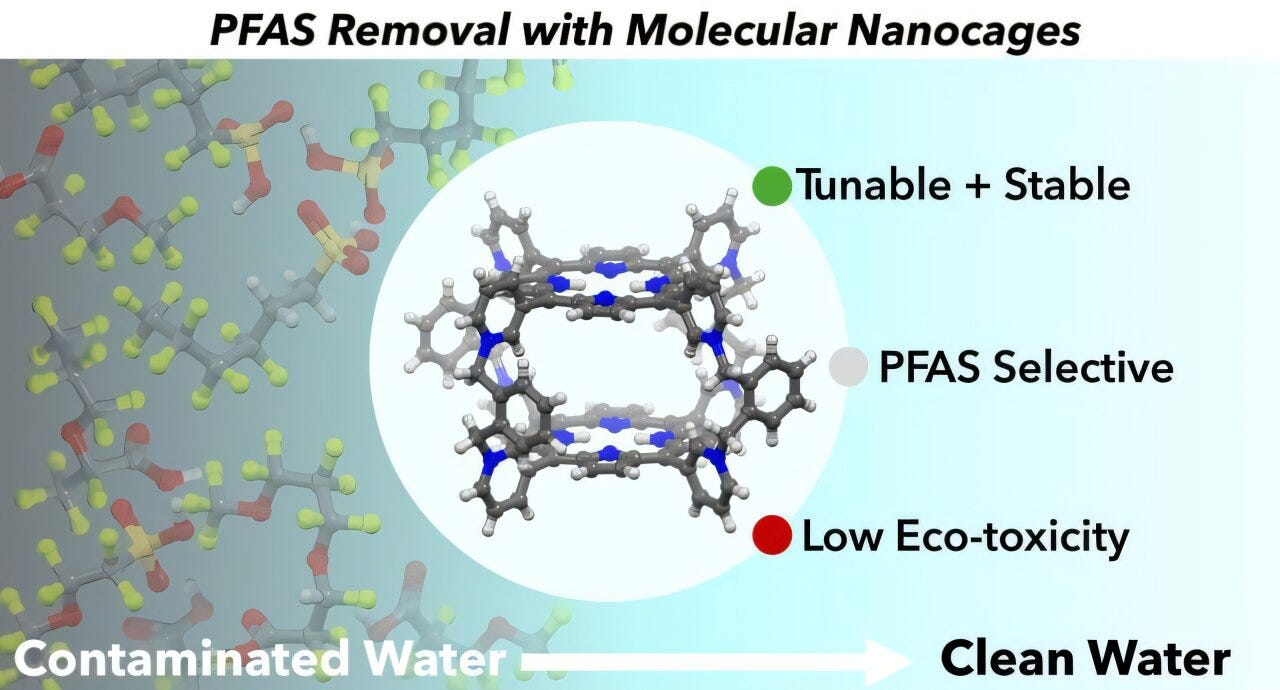
Molecular cages for PFAS capture
The final innovation for this week comes from the University of Buffalo. They created nano-sized cages to capture PFAS and remove it from water bodies.
Made of organic nanoporous material designed to capture only PFAS, this tiny chemical-based filtration system removed 80 to 90% of PFAS from sewage and groundwater during the study, respectively, while showing very low adverse environmental effects.The team was funded by the U.S. National Science Foundation, and you can read more about their work here. The researchers synthesized the nanocages from a group of organic chemicals called porphyrins. Previous studies have shown success with porphyrin nanocages in removing dyes, antibiotics, insecticides and chemicals that disrupt human hormone production from water. The organic molecular nanocages also outperformed the PFAS-filtering abilities of activated carbon, particularly in unprocessed sewage. They go on to say that the material can also be mass-produced at scale, and the cages are modifiable to remove PFAS only while leaving other water contents alone.
That is it for this Friday, until next week,
Peace!